Currently Empty: €0.00
Product Description
Precursor to other potassium compounds
Many potassium salts are prepared by neutralization reactions involving KOH. The potassium salts of carbonate, cyanide, permanganate, phosphate, and various silicates are prepared by treating either the oxides or the acids with KOH. The high solubility of potassium phosphate is desirable in fertilizers.
Manufacture of soft soaps
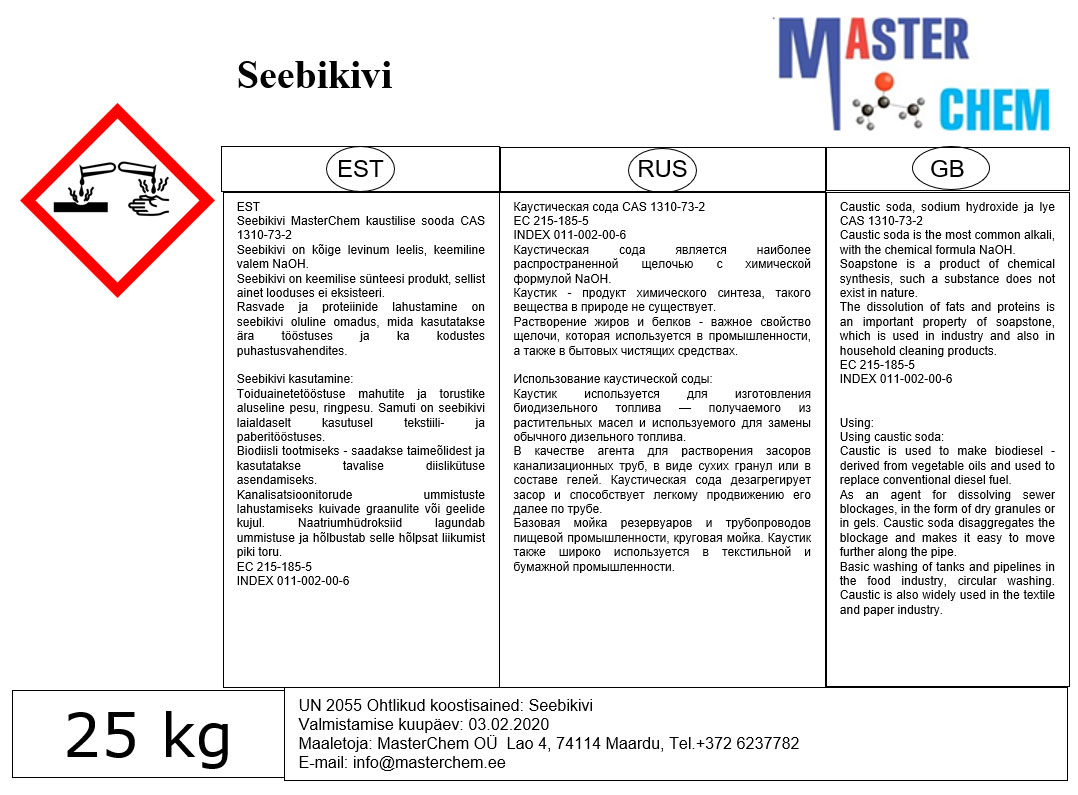
The saponification of fats with KOH is used to prepare the corresponding “potassium soaps”, which are softer than the more common sodium hydroxide-derived soaps. Because of their softness and greater solubility, potassium soaps require less water to liquefy, and can thus contain more cleaning agent than liquefied sodium soaps.
Food industry
In food products, potassium hydroxide acts as a food thickener, pH control agent and food stabilizer. The FDA considers it (as a direct human food ingredient) as generally safe when combined with “good” manufacturing practice conditions of use. It is known in the E number system as E525.
Niche applications
Like sodium hydroxide, potassium hydroxide attracts numerous specialized applications, virtually all of which rely on its properties as a strong chemical base with its consequent ability to degrade many materials. For example, in a process commonly referred to as “chemical cremation” or “resomation”, potassium hydroxide hastens the decomposition of soft tissues, both animal and human, to leave behind only the bones and other hard tissues. Entomologists wishing to study the fine structure of insect anatomy may use a 10% aqueous solution of KOH to apply this process.
In chemical synthesis, the choice between the use of KOH and the use of NaOH is guided by the solubility or keeping quality of the resulting salt.
The corrosive properties of potassium hydroxide make it a useful ingredient in agents and preparations that clean and disinfect surfaces and materials that can themselves resist corrosion by KOH.
KOH is also used for semiconductor chip fabrication. See also: anisotropic wet etching.
Potassium hydroxide is often the main active ingredient in chemical “cuticle removers” used in manicure treatments.
Because aggressive bases like KOH damage the cuticle of the hair shaft, potassium hydroxide is used to chemically assist the removal of hair from animal hides. The hides are soaked for several hours in a solution of KOH and water to prepare them for the unhairing stage of the tanning process. This same effect is also used to weaken human hair in preparation for shaving. Preshave products and some shave creams contain potassium hydroxide to force open the hair cuticle and to act as a hygroscopic agent to attract and force water into the hair shaft, causing further damage to the hair. In this weakened state, the hair is more easily cut by a razor blade.
Potassium hydroxide is used to identify some species of fungi. A 3–5% aqueous solution of KOH is applied to the flesh of a mushroom and the researcher notes whether or not the color of the flesh changes. Certain species of gilled mushrooms, boletes, polypores, and lichens are identifiable based on this color-change reaction.
| Dimensions | N/A |
|---|---|
| Pieejamais apjoms | 0,5 L metal bottle, 5 lit plastic can, 10 lit plastic can, 20 L plastic bucket/canister, 25 kg bag, 25 lit plastic can, 200 L (without container cost), 500 L (without container cost), 1000 L (without container cost) |