Currently Empty: €0.00
Nitric acid 56-58% (CAS 7697-37-2)
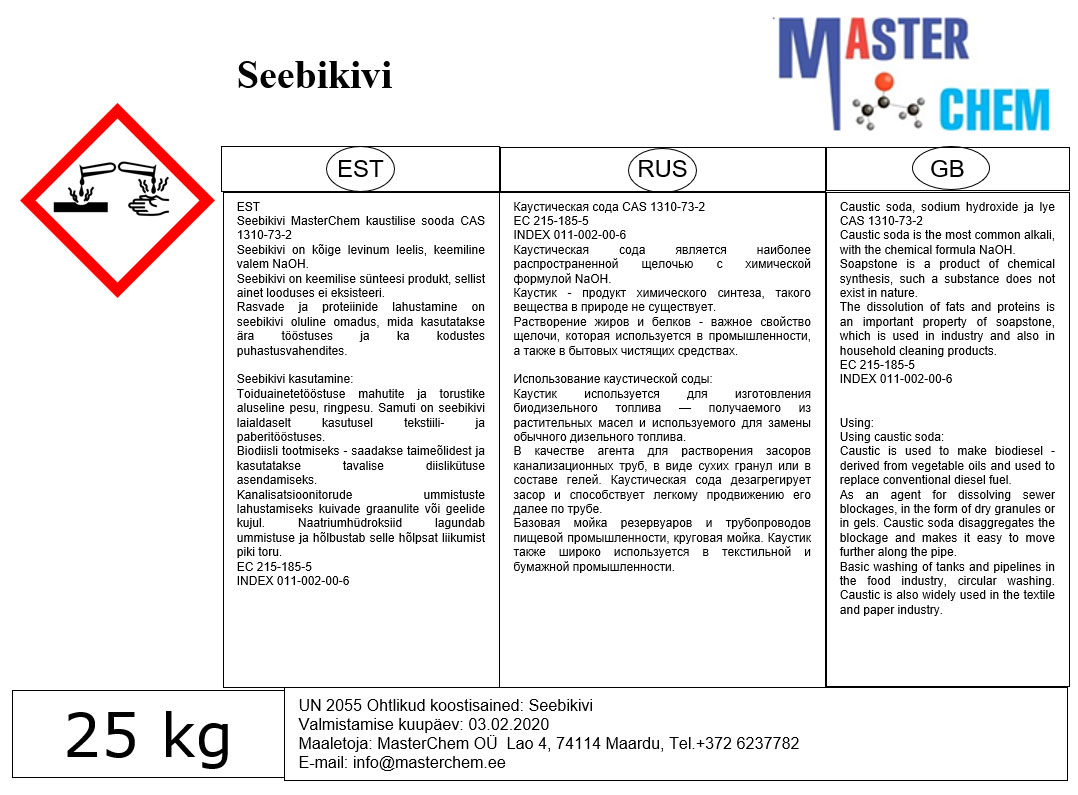
The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
CAS: 7697-37-2
€0.00 – €39.00
Product Description
Physical and chemical properties
Commercially available nitric acid is an azeotrope with water at a concentration of 68% HNO3. This solution has a boiling temperature of 120.5 °C at 1 atm. It is known as “concentrated nitric acid”. Pure concentrated nitric acid is a colourless liquid at room temperature.
Two solid hydrates are known: the monohydrate (HNO3·H2O or [H3O]NO3) and the trihydrate (HNO3·3H2O).
An older density scale is occasionally seen, with concentrated nitric acid specified as 42° Baumé.
Use as an oxidant
The precursor to nylon, adipic acid, is produced on a large scale by oxidation of “KA oil”—a mixture of cyclohexanone and cyclohexanol—with nitric acid.
Metal processing
Nitric acid can be used to convert metals to oxidized forms, such as converting copper metal to cupric nitrate. It can also be used in combination with hydrochloric acid as aqua regia to dissolve noble metals such as gold (as chloroauric acid). These salts can be used to purify gold and other metals beyond 99.9% purity by processes of recrystallization and selective precipitation.
Analytical reagent
In elemental analysis by ICP-MS, ICP-AES, GFAA, and Flame AA, dilute nitric acid (0.5–5.0%) is used as a matrix compound for determining metal traces insolutions. Ultrapure trace metal grade acid is required for such determination, because small amounts of metal ions could affect the result of the analysis.
It is also typically used in the digestion process of turbid water samples, sludge samples, solid samples as well as other types of unique samples which require elemental analysis via ICP-MS, ICP-OES, ICP-AES, GFAA and flame atomic absorption spectroscopy. Typically these digestions use a 50% solution of the purchased HNO
3 mixed with Type 1 DI Water.
In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes.
Woodworking
In a low concentration (approximately 10%), nitric acid is often used to artificially age pine and maple. The color produced is a grey-gold very much like very old wax- or oil-finished wood (wood finishing).
Etchant and cleaning agent
The corrosive effects of nitric acid are exploited for some specialty applications, such as etching in printmaking, pickling stainless steel or cleaning silicon wafers in electronics.
A solution of nitric acid, water and alcohol, Nital, is used for etching metals to reveal the microstructure. ISO 14104 is one of the standards detailing this well known procedure.
Nitric acid is used either in combination with hydrochloric acid or alone to clean glass cover slips and glass slides for high-end microscopy applications. It is also used to clean glass before silvering when making silver mirrors.
Commercially available aqueous blends of 5–30% nitric acid and 15–40% phosphoric acid are commonly used for cleaning food and dairy equipment primarily to remove precipitated calcium and magnesium compounds (either deposited from the process stream or resulting from the use of hard water during production and cleaning). The phosphoric acid content helps to passivate ferrous alloys against corrosion by the dilute nitric acid.
Nitric acid can be used as a spot test for alkaloids like LSD, giving a variety of colours depending on the alkaloid.
Safety
Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proteins (amide) and fats (ester), which consequently decomposes living tissue (e.g. skin and flesh). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin. These yellow stains turn orange when neutralized. Systemic effects are unlikely, however, and the substance is not considered a carcinogen or mutagen.
The standard first-aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water. Washing is continued for at least 10–15 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed immediately and the underlying skin washed thoroughly.
Being a strong oxidizing agent, nitric acid can react with compounds such as cyanides, carbides, or metallic powders explosively and with many organic compounds, such as turpentine, violently and hypergolically (i.e. self-igniting). Hence, it should be stored away from bases and organics.
| Weight | N/A |
|---|